Prof. P. A. Christensen
Home
Research and 3rd strand areas
News and recent publications
Vacancies
Information for group members
Clarizon Ltd
Services to business
Updated: 28 Jan 09
|
Recent in-situ FTIR data
The figure shows in-situ FT-IR spectra collected from a Pt/Ti electrode immersed in 0.5M CH3OH/0.1M H2SO4 at 20 C.
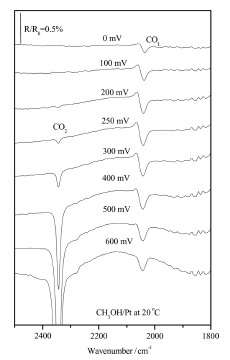
In-situ FTIR spectra collected from a Pt/Ti electrode immersed in 0.5M
CH3OH/0.1M H2SO4 at an initial potential of -200mV at room temperature of 20 C.
The potential was then stepped to 700mV in 50mV steps, with spectra taken at each
step. Some spectra are omitted for the sake of clarity. The reference spectrum was collected
at -200 mV.
The gain band
at 2343 cm-1 is attributed to the oxidation product CO2, and the ‘‘reversed’’ bipolar
band at around 2050 cm-1 (where the ‘‘gain’’ lobe is to lower frequency as the
potential is increased, which is the opposite behavior of that expected on the basis
of standard ‘‘electrochemical Stark effect’’ arguments (Christensen et al., 1999; Iwasita
and Nart, 1997); also see the above data obtained from the Ru(0001) electrode) may
be ascribed to linearly bonded CO (COL) adsorbed on the Pt nanoparticles and their
agglomerates (Lu et al., 2002; Chen et al., 2006).
The observation of the reversed bipolar band of COL was first reported by
Christensen et al. (1999) in their study of methanol oxidation on Pt particles supported
on carbon. It was explained by a potential-induced migration of adsorbed COL from
terraces to the edge or kink sites, and it was assumed that the CO stretching
frequency is higher for terrace sites than for edge or kink sites. Most recently, Sun
and his coworkers (Lu et al., 2002; Chen et al., 2006) have also observed reversed
(and enhanced) COLbands on Pt and other metal nanoparticles and their agglomerates
(supported on glassy carbon electrodes), and this phenomenon has been termed‘‘abnormal infrared effects’’ (AIREs) (Lu et al., 2002; Chen et al., 2006). The observation
of AIREs was explained by the strong interaction between Pt nanoparticles
and the collective interaction between CO adsorption and Pt nanoparticles inside
the agglomerates (Chen et al., 2006). Thus, it may be concluded from the figure that
Pt nanoparticles are present on the Pt/Ti electrode surface.
From: W. F. Lin; J. M. Jin; P. A. Christensen; F. Zhu; Z. G. Shao, “In-situ FTIR Spectroscopic Studies of Fuel Cell Electro-catalysis: From Single-crystal to Nano-particle Surfaces”, Chemical Engineering Communications, 195 (2008) 147 – 166.
Home
|



